À partir de son expertise historique en RMN quantitative de haute précision, MIMM a développé une expérience unique dans le développement de séquences d’impulsions originales pour l’analyse de mélanges complexes.
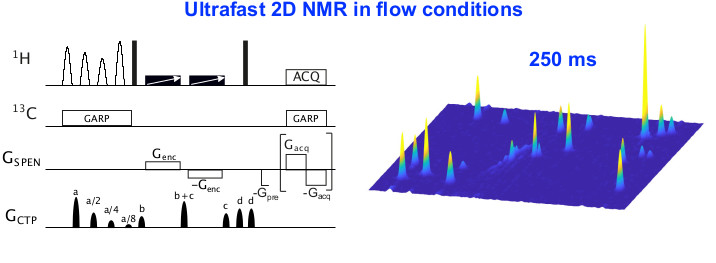
Nos principaux axes de recherche incluent le développement de méthodes quantitatives très précises basées sur des expériences multi-impulsionnelles et multi-dimensionnelles, et la conception de séquences d’impulsions mettant en œuvre un codage spatial de l’échantillon, telles que la RMN 2D ultrarapide pour l’analyse des mélanges hors équilibre.
Ces séquences d’impulsions sont implantées sur les spectromètres RMN haut champ de la plateforme RMN CEISAM, mais également sur des spectromètres RMN de paillasse récents équipés de bobines de gradients. Les développements incluent également des méthodes d’analyse des mélanges hyperpolarisés, à travers un prototype de système de polarisation nucléaire dynamique par dissolution installé en 2019.
Current team members: Margot Sanchez, , Gérald Remaud, Serge Akoka*.
Past team members: Sophie Renou, Tangi Jezequel, Valentin Joubert, Evelyne Baguet, Kévin Bayle, Ugo Bussy, Elsa Caytan, Flore Legrand, Eve Tenailleau, Christophe Thibaudeau.
This project is on-going as it is a permanent objective of the team from the beginnings. Isotopic 13C-NMR at natural abundance requires highly precise measurements (a few per mil), that have already been achieved by one-dimensional 13C acquisitions thank to an original 1H-decoupling scheme. However it is quite time consuming, even for a relatively simple molecular structure. Using optimized adiabatic multipulse sequences (1D or 2D) a dramatic reduction is gained in the experimental time without deterioration in short time or long-time stability. This approach permits to envisage for the first time 15N PSIA by NMR.
Until now, the specific compositions calculation required measurement of the global 13C composition (δ13Cg) by mass spectrometry. In a radical new approach, we have shown that an internal isotopic chemical reference can be used instead. The strategy uses 1H NMR to quantify molar concentrations in the NMR tube. Thus, the sample preparation protocol is greatly simplified, bypassing the previous requirement for precise purity and mass determination. The key to accurate results is suppressing the effect of radiation damping in 1H NMR which produces signal distortion and alters quantification.
Our efforts are now focused on increasing the SNR per unit time in 2H, so that we can perform 2H and 13C isotopic NMR measurements on the same instrument and probe. Strategies described in section "Acceleration of 1D experiments" will be used for this purpose.
The interest of oxygen isotopes content is of interest. No PSIA information are available on organic molecules. We currently working on 17O NMR to evaluate its capability.
References:
- C. Thibaudeau, G. Remaud, V. Silvestre, S. Akoka. Performance Evaluation of Quantitative Adiabatic 13C NMR Pulse Sequences for Site-specific Isotopic Measurements. Analytical Chemistry, 2010, 82 (13), 5582–5590.
- E. Martineau, S. Akoka, R. Boisseau, B Delanoue, P. Giraudeau. Fast quantitative 1H-13C 2D NMR with very high precision. Analytical Chemistry, 2013, 85(9) 4777-4783.
- T. Jezequel, V. Silvestre, K. Dinis, P. Giraudeau, S. Akoka. Optimized slice-selective 1H NMR experiments combined with highly accurate quantitative 13C NMR using an internal reference method. Journal of Magnetic Resonance, 2018, 289, 18-25.
- S. Akoka, G. Remaud. NMR-based isotopic and isotopomic analysis. Progress in NMR Spectroscopy, 2020, 120–121.
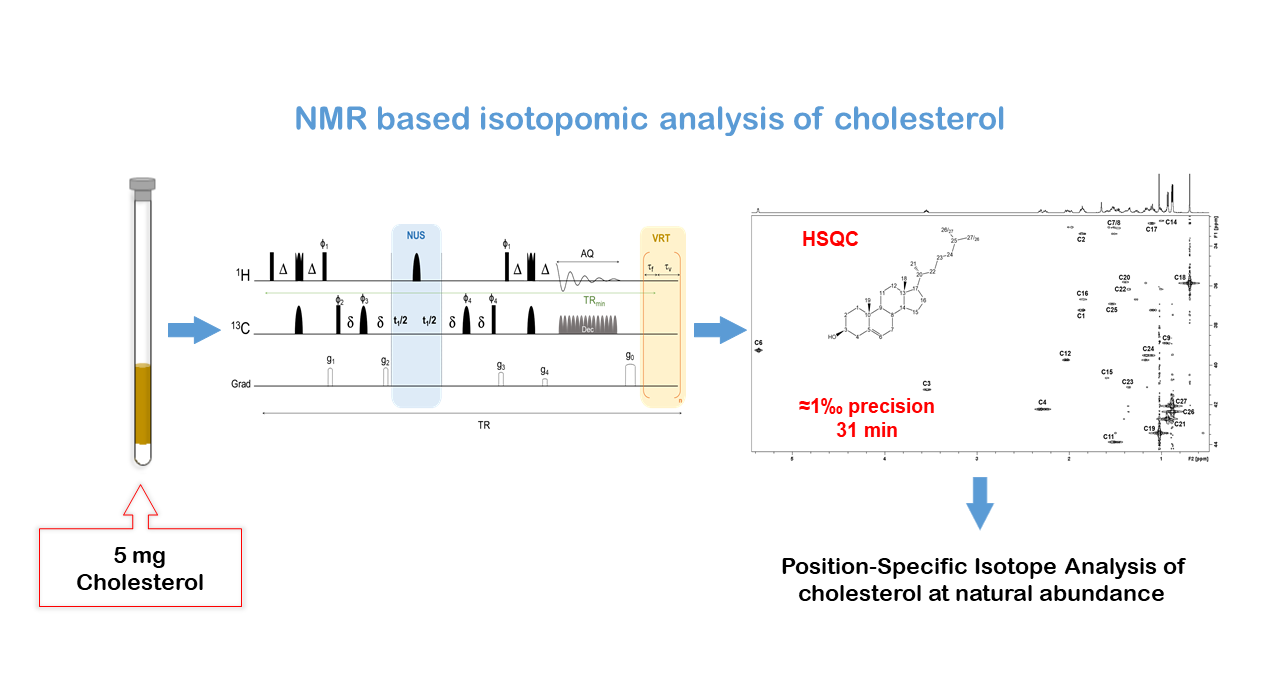
- L. Haddad, S. Renou, G. S. Remaud, T. Rizk, J. Bejjani, S. Akoka. A precise and rapid isotopomic analysis of small quantities of cholesterol at natural abundance by optimized 1H-13C 2D NMR. Analytical and Bioanalytical Chemistry 2021, 413, 1521–1532.
Supported by: National Council for Scientific research and Nantes Université
Current team members: Joris Mandral, Benoît Charrier, Jean-Nicolas Dumez, Patrick Giraudeau*, Jonathan Farjon*
Past team members: Shrikant Kunjir, Boris Gouilleux, Maxime Tharaud, Thomas Castaing-Cordier, Dylan Bouillaud
Collaborations (past and present): Fabrice Besacier and Virginie Ladroue (INPS Lyon), Paul Bowyer (Magritek USA), Ernesto Danieli and Juan Perlo (Magritek Germany), Myriam Malet-Martino (Univ. Toulouse).
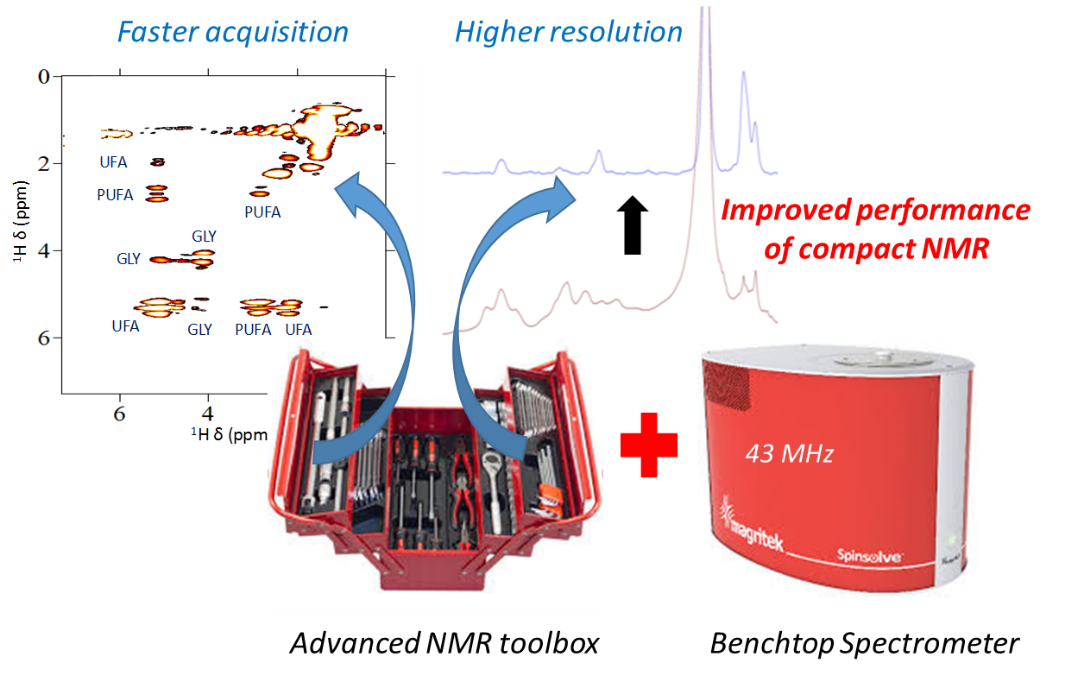
High-field NMR has been constantly improving with superconducting magnets and cold probes, but it is associated with high purchase and operating costs and the need for dedicated staff. In the last few years, a new generation of transportable and cryogen-free low-field spectrometers has emerged as a promising alternative. These permanent magnets have reached a recognized analytical potential with stable and homogeneous static magnetic fields.
However, those spectrometers operating at 1H resonance frequencies between 40 and 80 MHz involve a loss of analytical performance. In addition to the low sensitivity, the reduction of the spectral resolution leads to overcrowded spectra with huge signal overlaps, especially in the case of complex mixtures. Moreover, ubiquitous second order couplings make identification and quantification very difficult.
Thanks to the pulse sequence programming capabilities of benchtop spectrometers and to their instrumental improvement with a gradient coil, advanced high-field NMR methods have been successfully implemented to improve their analytical capabilities. Among them, we are particularly exploring spatially-encoded experiments such as ultrafast 2D NMR and pure-shift experiments. We have also demonstrated the potential of benchtop NMR DOSY to investigate complex mixtures. Gradient coils have also enabled the implementation of modern solvent suppression schemes. Applications to reaction and process monitoring are currently explored.
Key references:
- B. Gouilleux, B. Charrier, S. Akoka, F.-X. Felpin, M. Rodriguez-Zubiri, P. Giraudeau, Ultrafast 2D NMR on a benchtop spectrometer: Applications and perspectives, Trends Anal. Chem. 83, 65 (2016)
- B. Gouilleux, B. Charrier, S. Akoka, P. Giraudeau, Gradient-based solvent suppression methods on a benchtop spectrometer, Magn. Reson. Chem. 55, 91 (2017)
- T. Castaing-Cordier, D. Bouillaud, P. Bowyer, O. Gonçalves, P. Giraudeau, J. Farjon, Highly-resolved pure-shift spectra on a compact NMR spectrometer, ChemPhysChem, 20, 736 (2019)
- B. Gouilleux, J. Farjon, P. Giraudeau, Gradient-based pulse sequences for benchtop NMR spectroscopy J. Magn. Reson., 319, 106810 (2020)
- T. Castaing-Cordier, A. Benavides Restrepo, D. Dubois, V. Ladroue, F. Besacier, A. Buleté, C. Charvoz, A. Goupille, D. Jacquemin, P. Giraudeau, J. Farjon, Drug Test. Anal., 14, 1629 (2022).
Supported by Région Pays de la Loire (Paris scientifiques RésoNantes and AMER-METAL), CNRS (Projets interdisciplinarité RMN-(ME)2-TAL and Emergence ARES-TATION), and the French national research agency (ANR DEVIL_INSID).
Current team members: Estelle Martineau, Jérémy Marchand, Serge Akoka, Jonathan Farjon, Jean-Nicolas Dumez, Patrick Giraudeau*
Past team members: Tangi Jézéquel, Bertrand Plainchont, Boris Gouilleux, Adrien Le Guennec, Meerakhan Pathan, Laetitia Rouger
Collaboration (past and present): Stefano Caldarelli (Aix-Marseille Université), Philippe Lesot (ICMMO Orsay), Hassan Oulyadi (Rouen), Patricia Sepulcri (Sanofi Pasteur), Stanislav Sokolenko (Univ. Dalhousie), Christina Thiele (TU Darmstadt)
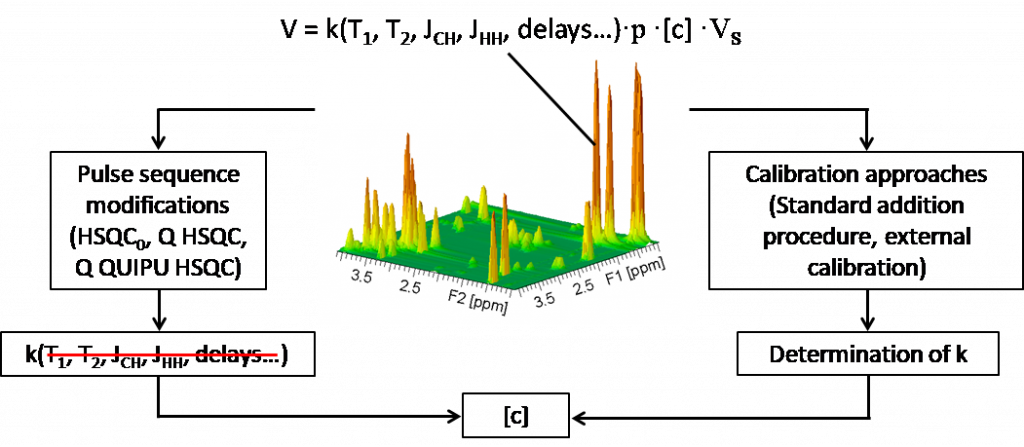
Measuring the accurate concentration of analytes in chemical or biochemical mixtures is, together with the identification of these components, one of the main goals of analytical chemistry. Nuclear Magnetic Resonance (NMR) is a widely used quantitative approach, known for its high reproducibility and robustness.
However, 1D proton NMR suffers from large overlap between peaks that restrains its quantitative use, particularly when complex mixtures are studied. In order to overcome these drawbacks, we are developing new quantitative 2D NMR approaches, since multidimensional NMR offers a better discrimination between resonances in complex samples with overlapped peaks.
Still, conventional 2D NMR experiments are affected by long experiment durations that, beyond timetable constraints, limit their use for quantitative analysis (since long experiments are highly sensitive to spectrometer instabilities) or for studying fast processes (kinetics, dynamics, etc.). For this reason, we focus on developing fast and precise 2D NMR experiments and we apply them to a variety of quantitative studies. A large part of our projects concerns the development of quantitative methods based on the ultrafast 2D NMR methodology, which makes it possible to record 2D NMR spectra in a fraction of a second to a few minutes.
Key References:
- B. Gouilleux, L. Rouger, P. Giraudeau, Chapter Two - Ultrafast 2D NMR: Methods and Applications, in: G.A. Webb (Ed.) Annual Reports on NMR Spectroscopy, Academic Press, 75 (2018)
- J. Farjon, C. Milande, E. Martineau, S. Akoka, P. Giraudeau, The FAQUIRE Approach: FAst, QUantitative, hIghly Resolved and sEnsitivity Enhanced 1H, 13C Data, Anal. Chem. 90, 1845 (2018)
- P. M. Le, C. Milande, E. Martineau, P. Giraudeau, J. Farjon, Quantification of natural products in herbal supplements: A combined NMR approach applied on goldenseal, J. Pharm. Biomed. Anal., 165, 155 (2019).
- P. Giraudeau, Quantitative NMR spectroscopy of complex mixtures, Commun. 59, 6627 (2023).
Supported by: ERC (CoG SUMMIT 814747), ANR (QUANTUM and T-ERC SUMMIT), IUF, industrial funding
Current team members: Victor Ribay, Benoît Charrier, , Marine Letertre, Jean-Nicolas Dumez*, Patrick Giraudeau*
Past team members: Arnab Dey, Clément Praud, Bertrand Plainchont, Elodie Lesquin
Collaborations (past and present): Geoffrey Bodenhausen (ENS Paris), Catherine Deborde and Annick Moing (INRAe Bordeaux), Lucio Frydman (Weizmann Institute, Rehovot), Sami Jannin (Univ. Lyon), James Kempf and Dmitry Eshchenko (Bruker Biospin), Mikaël Croyal (M-SHARK Nantes).
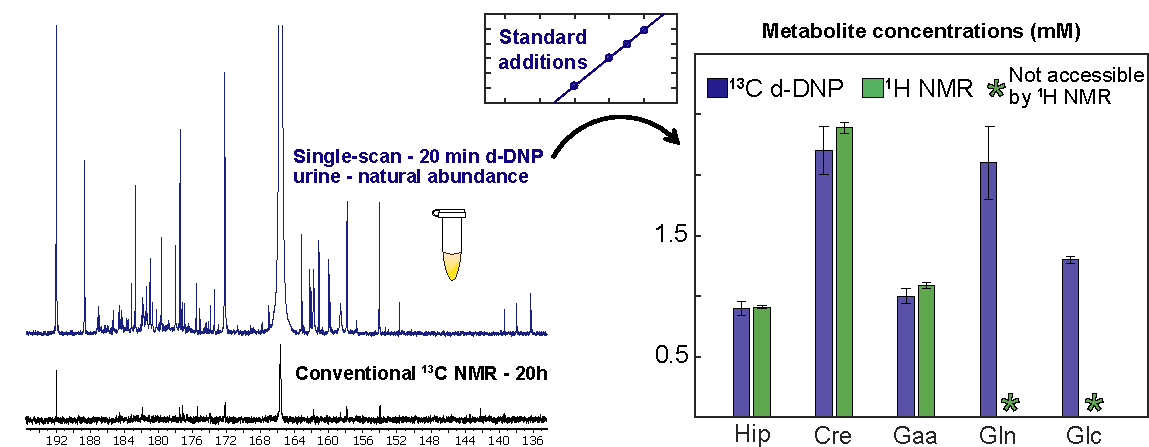
First d-DNP-enhanced 13C NMR analysis of a biofluid -urine- at natural abundance, offering unprecedented resolution and sensitivity for this challenging type of sample
Hyperpolarization methods can boost the sensitivity of NMR by several orders of magnitude. In particular, dissolution dynamic nuclear polarization (D-DNP) can yield sensitivity improvements by 4 to 5 orders of magnitude in liquid-state NMR. While D-DNP has been widely applied to in vivo situations, its application to analytical chemistry, and in particular to the analysis of complex mixtures, remains limited. We are exploring the potential of D-DNP in this context, with the aim to improve its applicability to “omics” applications.
Thanks to a careful optimization of a D-DNP prototype installed at CEISAM in 2019 (in collaboration with Bruker Biospin and Universite de Lyon), we recently demonstrated that D-DNP assisted by cross-polarization can provide major sensitivity enhancements in samples as complex as extracts or biofluids (urine), with a repeatability of a few percent.. We also showed that D-DNP can be used in untargeted metabolomics workflows or for targeted quantitative analysis (when combined with standard additions).
Key References:
- A. Dey, B. Charrier, E. Martineau, C. Deborde, E. Gandriau, A. Moing, D. Jacob, D. Eschenko, M. Schnell, R. Melzi, D. Kurzbach, M. Ceillier, Q. Chappuis, S.F. Cousin, J.G. Kempf, S. Jannin, J.-N. Dumez, and P. Giraudeau, Hyperpolarized NMR Metabolomics at Natural 13C Abundance, Anal. Chem. 92, 14867 (2020)
- A. Dey, B. Charrier, K. Lemaitre, V. Ribay, D. Eshchenko, M. Schnell, R. Melzi, Q. Stern, S. F. Cousin, J. G. Kempf, S. Jannin, J. N. Dumez, P. Giraudeau, Fine optimization of a dissolution dynamic nuclear polarization experimental setting for 13C NMR of metabolic samples, Magn. Reson., 3, 183 (2022).
- V. Ribay, A. Dey, B. Charrier, C. Praud, J. Mandral, J.-N. Dumez, M. P. M. Letertre, P. Giraudeau, Hyperpolarized 13C NMR Spectroscopy of Urine Samples at Natural Abundance by Quantitative Dissolution Dynamic Nuclear Polarization, 62, e202302110 (2023).
- C. Praud, V. Ribay, A. Dey, B. Charrier, J. Mandral, J. Farjon, J.-N. Dumez, P. Giraudeau, Optimization of heteronuclear ultrafast 2D NMR for the study of complex mixtures hyperpolarized by dynamic nuclear polarization, Anal Methods, 2023, 15, 6209 (2023).
- A. Dey, B. Charrier, V. Ribay, J.-N. Dumez, P. Giraudeau, Hyperpolarized 1H and 13C NMR Spectroscopy in a Single Experiment for Metabolomics., Anal. Chem, 2023, 95, 16861 (2023)
Supported by: ERC (CoG SUMMIT 814747), ANR (MetaboHub, T-ERC SUMMIT), IUF, PRC CNRS-MOST
Current team members: Margherita Bazzoni, Yuliia Horbenko, Armand Régheasse, Aurélie Bernard, Patrick Giraudeau and Jean-Nicolas Dumez*
Past team members: Arnab Dey, Ludmilla Guduff, Ghanem Hamdoun , Corentin Jacquemmoz, Célia Lhoste, Benjamin Lorandel, Achille Marchand, Rituraj Mishra, Clément Praud
Collaborations (past and present): Daniel Abergel (ENS, Paris), Mohammed Boujtita (Univ. Nantes), Luiz Keng Queiroz and Antonio Gilberto Ferreira (Univ. Sao Carlos), François-Xavier Felpin (Nantes Université), Lucio Frydman (Weizmann Institute), Vincent Gandon (Université Paris-Saclay), Gaspard Huber (CEA, Saclay), Sami Jannin (Université de Lyon), Dennis Kurzbach (University of Vienna), Géraldine Masson (ICSN, Univ. Paris-Saclay), Anna Parker (NVision Imaging)
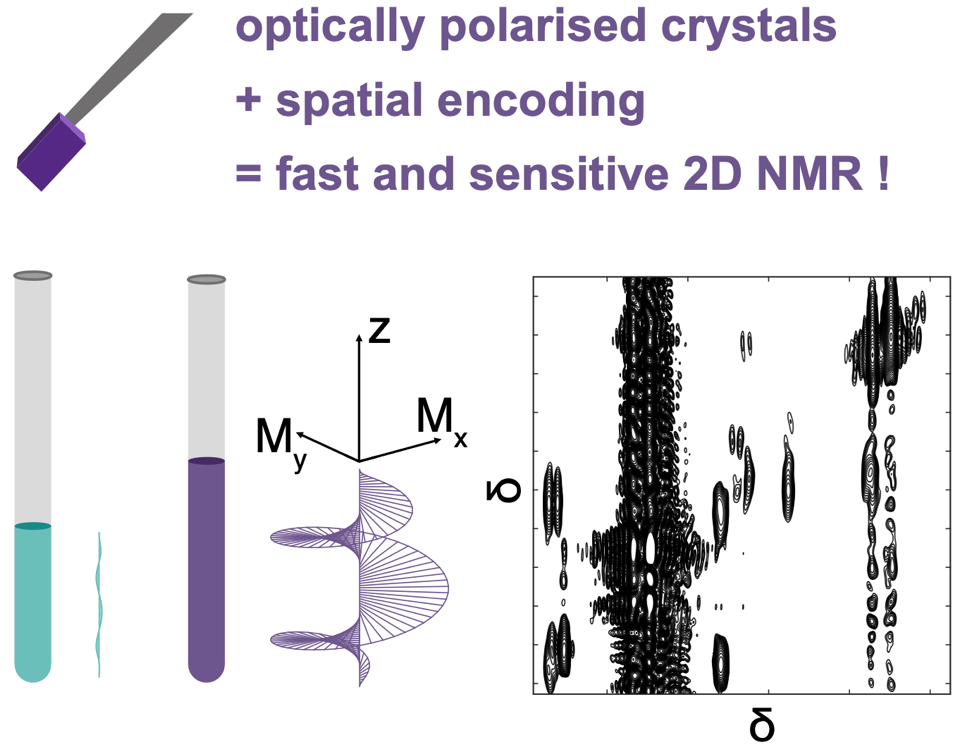
Solution mixtures that evolve in time encompass a broad range of samples and applications, such as the monitoring of organic and catalytic chemical reactions or the sensitive detection of components in hyperpolarized samples. Multidimensional (ND) NMR spectroscopy can provide extensive information on static solution mixtures, but classic ND experiments typically require several minutes or more, and are often not robust against sample evolution during the measurement.
We develop fast multidimensional NMR methods that are compatible with the analysis of time-evolving samples, with three complementary objectives: i/ reducing the duration needed to collect a complete ND data set (using notably single-scan “ultrafast” 2D NMR; ii/ optimising the frequency with which a time-evolving system can be sampled (with polarisation saving methods and original hardware); iii/ making each measurement more robust against sources of errors, such as convection of flow. The resulting methods open new perspective for mechanistic studies in chemical synthesis and metabolomics applications.
Recent references:
- C. Jacquemmoz, F. Giraud and J.-N. Dumez, Online reaction monitoring by single-scan 2D NMR under flow conditions, Analyst145, 478 (2020).
- K. Singh, C. Jacquemmoz, P. Giraudeau, L. Frydman, and J.-N. Dumez, Ultrafast 2D 1H–1H NMR spectroscopy of DNP-hyperpolarised substrates for the analysis of mixtures, Chem. Commun. 57, 8035 (2021).
- A. Marchand, R. Mishra, A. Bernard and J.-N. Dumez, Online Reaction Monitoring with Fast and Flow-Compatible Diffusion NMR Spectroscopy, Chem. Eur. J. e202201175 (2022).
- C. Lhoste, B. Lorandel, C. Praud, A. Marchand, R. Mishra, A. Dey, A. Bernard, J.-N. Dumez, P. Giraudeau, Ultrafast 2D NMR for the analysis of complex mixtures, Prog. Nucl. Magn. Reson. Spectrosc. 130, 1 (2022).
- C. Lhoste, M. Bazzoni, J. Bonnet, A. Bernard, F.-X. Felpin, P. Giraudeau, and J.-N. Dumez, Broadband ultrafast 2D NMR spectroscopy for online monitoring in continuous flow, Analyst 148, 5255 (2023).
- C. Praud, V. Ribay, B. Charrier, J. Mandral, J. Farjon, J.-N. Dumez, and P. Giraudeau, Optimization of heteronuclear ultrafast 2D NMR for the study of complex mixtures hyperpolarized by dynamic nuclear polarization, Anal. Methods 15, 6209 (2023).
- A. J. Parker, A. Dey, M. U. Qureshi, J. M. Steiner, J. W. Blanchard, J. Scheuer, N. Tomek, S. Knecht, F. Josten, C. Müller, P. Hautle, I. Schwartz, P.Giraudeau, T. R. Eichhorn, and J.-N. Dumez, Solution-State 2D NMR Spectroscopy of Mixtures Hyperpolarized Using Optically Polarized Crystals, Angew. Chem. Intl. Ed., z202312302 (2023)
Supported by: Agence Nationale de la Recherche (PRC DigitalChem, PRC SOFTNMR), Région Pays de la Loire (Connect Talent HPNMR), Royal Society, and European Research Council (StG DINAMIX, CoG SUMMIT)
Current team members: Margherita Bazzoni, Armand Régheasse, Aurélie Bernard, Patrick Giraudeau*, and Jean-Nicolas Dumez*
Past team members: , Marion André, Oksana. Cazimajou, Maria Grazia Concilio, Boris Gouilleux, Ludmilla Guduff, Adrien Le Guennec, Corentin Jacquemmoz, Célia Lhoste, Benjamin Lorandel, Rituraj Mishra, Bertrand Plainchont, Laetitia Rouger.
Collaborations (past and present): Damien Jeannerat (Université de Genève), Ilya Kuprov (University of Southampton), Vincent Sarou-Kanian and Franck Fayon (CNRS Orléans)
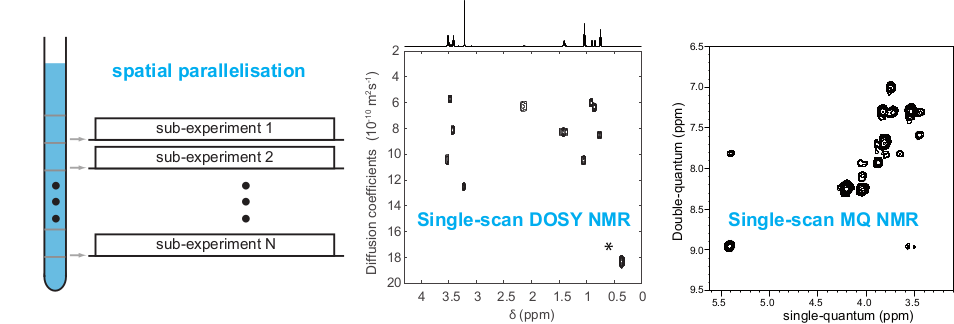
The potential of NMR spectroscopy can be greatly enhanced by introducing concepts and methods that exploit spatial (in addition to spectral) dimensions, often building on magnetic resonance imaging (MRI) knowledge and tools. Spatial parallelisation strategies notably yield 2D NMR spectra in a single-scan, and provide a powerful route to the acquisition of ultrahigh resolution 1H spectra. It can also accelerate the acquisition of ultraselective experiments, that target 1H multiplets that overlap with other multiplets. We develop novel strategies for the spatial encoding of NMR interactions and other physical properties, such as translational diffusion coefficients.
These developments jointly rely on the experimental, theoretical and numerical characterisation of the underlying spin dynamics. Specifically, we develop pulse sequence elements for spatial encoding (SPEN) (of, e.g., chemical shifts or diffusion coefficients), and integrate them into accelerated experiments for mixture analysis, with the goals of broadening the range of systems that can be analysed, and increasing the information content of NMR spectra. These basic ingredients are then used in all of our mixture analysis projects.
Recent references:
- B. Plainchont, P. Giraudeau and J.-N. Dumez, Interleaved spatial/spectral encoding in ultrafast 2D NMR spectroscopy, J. Magn. Reson.305, 112 (2019).
- J-N. Dumez, Frequency-swept pulses for ultrafast spatially encoded NMR, J. Magn. Reson. 323, 106817 (2020).
- Jacquemmoz, R. Mishra, L. Guduff, C. van Heijenoort, and J.-N. Dumez, Optimisation of spatially-encoded diffusion-ordered NMR spectroscopy for the analysis of mixtures, Magn Reson. Chem. 60, 121 (2022).
- R. Mishra, A. Marchand, C. Jacquemmoz, and J.-N. Dumez, Ultrafast diffusion-based unmixing of 1H NMR spectra, Chem. Commun. 57, 2384 (2021).
- R. Mishra and J.-N. Dumez, Quadratic spacing of the effective gradient area for spatially encoded diffusion NMR, J. Magn. Reson. 334, 107114 (2022).
- R. Mishra and J.-N. Dumez, Theoretical analysis of flow effects in spatially encoded diffusion NMR, J. Chem. Phys. 158, 014204 (2023)
- B. Lorandel, R. Mishra, O. Cazimajou, A. Marchand, A. Bernard, and J.-N. Dumez, Accounting for gradient non-uniformity in spatially-encoded diffusion-ordered NMR spectroscopy, J. Magn. Reson. 355, 207543 (2023)
- M. Bazzoni, R. Mishra, and J.-N. Dumez, Single-Scan Ultraselective NMR Experiments with Preserved Sensitivity, Angew. Chem. Intl. Ed. e202314598 (2023)
Supported by: Agence Nationale de la Recherche (PRC SOFTNMR), Région Pays de la Loire (Connect Talent HPNMR), Royal Society, and European Research Council (STG DINAMIX, CoG SUMMIT)
Current team members: Margot Sanchez, Serge Akoka*
Collaboration (past and present): Gaëtan Assemat (RS2D), Klemens Kessler (QUAD Systems)
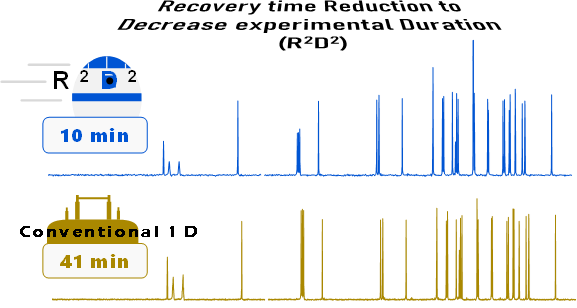
Acceleration techniques are essential in most of the application domains of 1 D NMR. Furthermore, for chemists that use it for structural elucidation, such techniques need to be straightforward in routine.
In order to reach this goal, we proposed a new approach. Recovery time Reduction to Decrease the experimental Duration (R2D2) relies on the incremental reduction of pulse sequence’s Recycle Time (TR). It requires only a list of recovery times and 2D processes to operate, it is thus easily adaptable on any device without any additional cost. With this method, we were able to easily reduce the experimental time by a factor of 2 and up to 4 using single-pulse, APT and DEPT 13C sequences. Moreover, R2D2 has the potential to be used on other low abundance nuclei (such as 15Nor 2H) or other domains as solid-state NMR, and it can be combined with many sequences, notably in order to increase the acceleration factor.
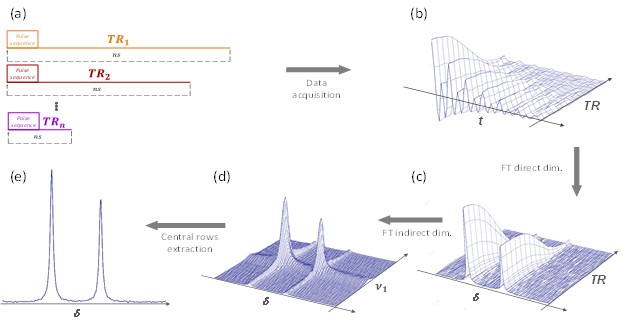
The R2D2 Method can be summarized as follows. The pulse sequence is repeated ns times with a specific Repetition Time TRi before moving on to another acquisition with the same number of repetitions but a different repetition time, TRi+1, slightly smaller than the previous one. Acquired data have 2 dimensions: the FID of the desired spectrum is in the direct (time) dimension, while in the indirect (TR) dimension, there is the signal evolution according to TR, starting with TR1. Fourier Transform in the indirect dimension will generates peaks on the central frequency. 1 D spectrum is obtained by addition of central rows, as these are the ones that have the largest intensity.
Key References:
- M. Sanchez, J. Pontabry, G. Assemat, A. Martinez, S. Akoka. Recovery time Reduction to Decrease experimental Duration (R2D2): a simple and universal method to accelerate NMR experiments, Anal. Chem. submitted (2023)
Supported by: ANRT, RS2D, QUAD Systems

