L’interaction lumière/matière est à l’origine de nombreuses propriétés qui ouvrent la porte à des applications très variées. Par exemple, la lumière du soleil est une source gigantesque d’énergie non polluante et les projets que développés par l'équipe dans le domaine des cellules photovoltaïques à colorant et de la photosynthèse artificielle visent justement à exploiter cette formidable manne énergique.
L’énergie lumineuse emmagasinée par la matière après absorption peut également servir à promouvoir l’activité catalytique d’un système fondé sur des nanoparticules métalliques ou dépolluer les eaux sans utilisation de détergeant ; ce qui représente un autre axe développé dans IMF. Au-delà de l’aspect énergétique du photon, la lumière constitue également un élément d’information exploité par l'équipe pour écrire sur la matière afin de stocker de l’information avec une densité très élevée.
PI: Fabrice ODOBEL
As compared to the much extensively developed silicon based solar cells, dye sensitized solar cells (DSSCs) present the discriminating advantages of having a shorter energy payback time, the color of the cell is tunable over all the rainbow, they are transparent and aesthetic, compatible with flexible substrates, they are lightweight and very importantly they display higher performances under low light conditions and particularly under artificial lightning. For example, Hagfeldt and co-workers report an efficiency up to 28.9% under indoor illumination with a model Osram 930 warm-white fluorescent light tube (1000 lux), which even outperforms a GaAs thin-film solar cell in the same conditions.1 Accordingly, DSSCs represent an attractive solution to produce electricity with low cost and is a valuable technology for building integrated photovoltaic (BIPV) or for indoor applications. The works on DSSCs is also driven by the possible implementation of these photoelectrodes to design dye sensitized photoelectrosynthetic cells (DSPECs) for solar fuels, another area of our interest (see artificial photosynthesis projects). Basically, our works on DSSCs focus on two main directions:
1. Convention n-type dye sensitized solar cells (n-DSSCs) based on TiO2
There are two different research domains in the area of DSSCs. In the first one, the cells are based on the sensitization of an n-type semi-conductor (essentially TiO2) and are better known as “Grätzel cells” and there have been many extensive studies on this field. In this first area, our interest was directed towards two main topics: i) amplification of the light collection by antenna effect and ii) the development of organic dyes for colorless and transparent solar cells.The second area (p-DSSC), developed more recently, concerns the sensitization of a p-type semiconductor (typically NiO) by a dye and we have been a major contributor in this field.
1.1 Light collection by antenna applied to DSSCs
In plant and photosynthetic organisms sunlight is collected by a large number of chromophores called Light Harvesting Antenna (LHA) embedded around the Photosynthetic Reaction Center.2 The LHA enables to achieve a large absorption cross section thanks to the numerous molecular absorbers, composed of chlorophyll derivatives and carotenoids, having complementary absorption spectra. The fantastic property of the LHA ensures that each photon absorbed by any chromophore in the LHA is funneled to the Photosynthetic Reaction Center with a quantum efficiency close to unity.
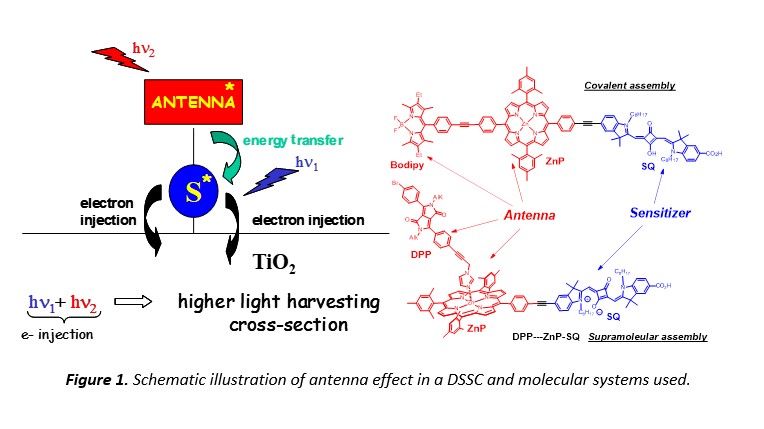
The first step of any photovoltaic process is to collect photons, accordingly it appears logical to get inspiration from the strategy developed by Nature over billion year of evolution to optimize this function. In other words, we have prepared multi-chromophoric array to maximize the absorption cross-section of a DSSC (Figure 1). The role of the antennas is to collect photons where the sensitizer is a poor absorber and to transfer the energy to the sensitizer (Figure 1).3 Towards this objective, we have appended supplementary chromophores playing the role of antennas such as a boroazaindacene (Bodipy), a zinc porphyrin (ZnP) or a diketopyrrolopyrrole (DPP) linked to a squaraine (SQ) sensitizer (Figure 1). The supplementary chromophores (antennas) can be connected to the sensitizer either by covalent bond4, 5 or by supramolecular interaction.6, 7
1.2 Colorless and transparent DSSCs
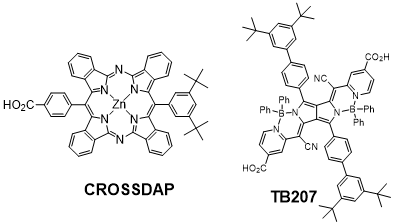
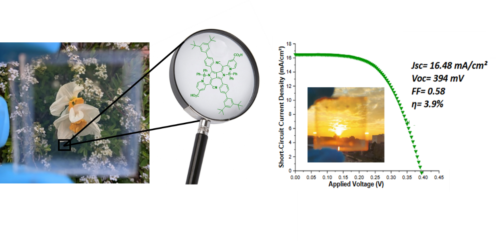
Visibly transparent solar cells is an emerging concept, which has garnered increasing recent scientific attention. It can afford significant energy production by providing electricity via transparent and colorless windows in buildings and cars. Moreover, colorless sensitizers can constitute valuable complementary dyes to fabricate panchromatic DSSC, which would exploit sunlight from ultraviolet (UV) to the near infrared (NIR). Towards this application, we have designed and prepared original dyes presenting high transmission in the visible region (400-700 nm), but high absorbance in the UV and more particularly in the NIR (> 700 nm), where the flux of photons is significant. Several families of dyes are currently explored toward this end; among them diazabenzoporphyrines (CROSSDAP)9 and pyrrolopyrrole cyanine10 derivatives, because they display intense absorption bands situated in the NIR and are very active in DSSCs (Figures 2).
2. Inverted p-type dye sensitized solar cells (p-DSSCs) based on the sensitization of a wide bandgap semiconductor such as NiO
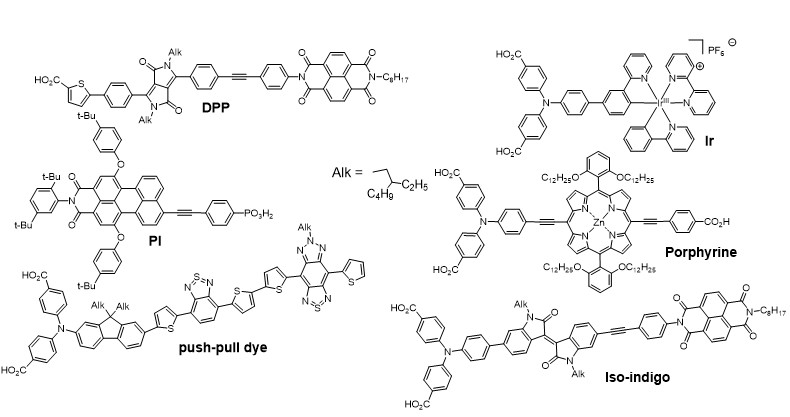
Apart from classical n-DSSCs, there is a second category of DSSCs, much less documented, based on the sensitization of a p type wide bandgap inorganic semi-conductor (usually NiO). The operation principle of a pDSSC is based on the photo-injection of a hole from the sensitizer into the valence band of the p-SC (mainly NiO).11, 12, 13 We have importantly contributed to this much less investigated research field, by developing organic and quantum dot photosensitizers, analyzing their physical properties by absorption and emission spectroscopies and electrochemistry, and finally building and evaluating our own photovoltaic devices. This was possible, thanks to the precious time-resolved photophysical studies thanks to a long time collaboration with Pr. Leif Hammarström (Uppsala Sweden) and more recently with Prof. Eric Vauthey (Geneva University). More precisely, we have developed various classes of organic dyes for this application such as perylene imides (PI),14, 15 porphyrines,16, 17 diketopyrrolopyrroles (DPP),18, 19 push-pull dyes,20, 21 and also iridium24, 25 and ruthenium25, 26 polypyridine complexes (Figure 3) along with inorganic materials such as PbS quantum dots22, 23 (Figure 4).
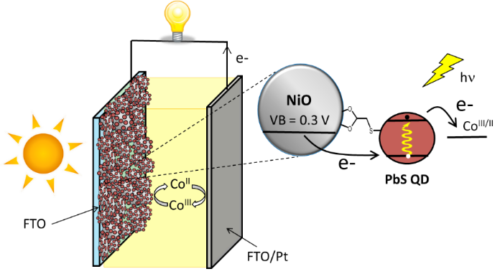
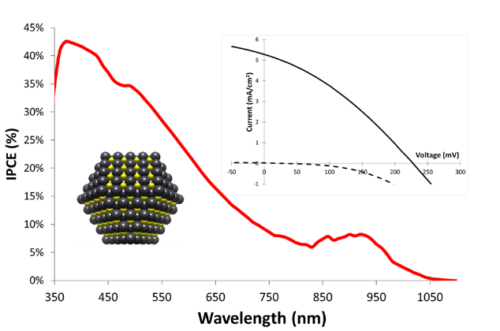
NiO is the most investigated p-SC in p-DSSCs, but it presents many drawbacks,13 which prompted the scientific community to explore alternative oxides with p-type semi-conductivity. In this context, in collaboration with Prof. Stéphane Jobic at IMN (Institut des Matériaux Jean Rouxel) we have shown that delafossites such as CuGaO2 are other copper based oxides are promising substitutes of NiO, because they are less colored materials and their valence band is deeper giving thus a higher photovoltage.27, 28, 29, 30, 31
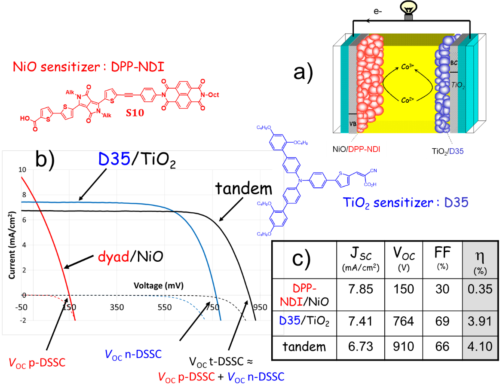
Finally, the photocathodes made of p-type sensitized SCs provide the entry into the field of tandem dye sensitized solar cell (t-DSSC), since the latter can be connected to a TiO2 photoanode to build a two absorbers solar cell, whose performances could be greater than those of single absorber DSSC. In this area, we have prepared the most efficient tandem DSSC reported so far and whose efficiency is higher than that of the individual sub-cell (Figure 5).32
Major collaboration: Prof. Leif Hammarström (Uppsala Univ, Suède) ; Prof. Eric Vauthey (Université de Genève) ; Prof. Denis Jacquemin (CEISAM, Nantes) ; Prof. Stéphane Jobic (IMN, Nantes) ; Prof. Dave Rogers. (Nanovation: private company at Chateaufort), Prof. Dmitry Aldakov (CEA, Spram, Grenoble), Prof. Frederic Sauvage (Université d’Amiens) ; Prof. Stefan Haacke (Université de Strasbourg), Prof. Mahfoudh Raissi (Kelenn Technology: private company at Igny).
Financial supports: Project POSITIF (ANR Progelec, 2012-2017) ; Project QuePhelec (ANR blanc, 2013-2017) ; Project Vision-NIR (ANR, 2018-2022), LUMOMAT (first financially supported by Région Pays de la Loire and now by EUR).
References :
- M. Freitag; J. Teuscher; Y. Saygili; X. Zhang; F. Giordano; P. Liska; J. Hua; S. M. Zakeeruddin; J.-E. Moser; M. Grätzel; A. Hagfeldt, "Dye-sensitized solar cells for efficient power generation under ambient lighting." Nature Photonics, 2017, 11, 372, http://dx.doi.org/10.1038/nphoton.2017.60.
- G. McDermott; S. M. Prince; A. A. Freer; A. M. Hawthornthwaite-Lawless; M. Z. Papiz; R. J. Cogdell; N. W. Isaacs, "Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria." Nature, 1995, 374, 6522, 517-521, http://dx.doi.org/10.1038/374517a0
- F. Odobel; Y. Pellegrin; J. Warnan, "Bio-inspired artificial light-harvesting antennas to enhance solar energy capture in dye-sensitized solar cells." Energy Environ. Sci., 2013, 6, 7, 2041-2052, http://dx.doi.org/10.1039/C3EE24229C.
- J. Warnan; L. Favereau; F. Meslin; M. Severac; E. Blart; Y. Pellegrin; D. Jacquemin; F. Odobel, "Diketopyrrolopyrrole–Porphyrin Conjugates as Broadly Absorbing Sensitizers for Dye-Sensitized Solar Cells." ChemSusChem, 2012, 5, 8, 1568-1577, http://dx.doi.org/10.1002/cssc.201100764.
- J. Warnan; F. Buchet; Y. Pellegrin; E. Blart; F. Odobel, "Panchromatic Trichromophoric Sensitizer for Dye-Sensitized Solar Cells Using Antenna Effect." Org. Lett., 2011, 13, 15, 3944-3947, http://pubs.acs.org/doi/abs/10.1021/ol2014686
- J. Warnan; Y. Pellegrin; E. Blart; F. Odobel, "Supramolecular light harvesting antenna to enhance absorption cross-section in dye-sensitized solar cells." Chem. Commun., 2012, 48, 5, 675-677, https://pubs.rsc.org/en/content/articlelanding/2012/cc/c1cc16066d#!divAbstract.
- G. Charalambidis; K. Karikis; E. Georgilis; B. L. M'Sabah; Y. Pellegrin; A. Planchat; B. Lucas; A. Mitraki; J. Boucle; F. Odobel; A. G. Coutsolelos, "Supramolecular architectures featuring the antenna effect in solid state DSSCs." Sustainable Energy & Fuels, 2017, 1, 2, 387-395, http://dx.doi.org/10.1039/C6SE00051G.
- J. Fortage; E. Göransson; E. Blart; H.-C. Becker; L. Hammarström; F. Odobel, "Strongly coupled zinc phthalocyanine-tin porphyrin dyad performing ultra-fast single step charge separation over a 34 Ã distance." Chem. Commun. 2007, 44, 4629-4631, https://pubs.rsc.org/en/content/articlelanding/2007/CC/b711642j#!divAbstract.
- D. S. Andrianov; Y. Farre; K. J. Chen; J. Warnan; A. Planchat; D. Jacquemin; A. V. Cheprakov; F. Odobel, "Trans-disubstituted benzodiazaporphyrin: A promising hybrid dye between porphyrin and phthalocyanine for application in dye-sensitized solar cells." J. Photochem. Photobiol., A, 2016, 330, 186-194, https://www.sciencedirect.com/science/article/pii/S1010603016303215.
- Baron, T.; Naim, W.; Nikolinakos, I.; Andrin, B.; Pellegrin, Y.; Jacquemin, D.; Haacke, S.; Sauvage, F.; Odobel, F. Transparent and Colorless Dye-Sensitized Solar Cells Based on Pyrrolopyrrole Cyanine Sensitizers. Angew. Chem. Inter. Ed., 2022, 61, 35, e202207459, https://doi.org/10.1002/anie.202207459.
- F. Odobel; L. Le Pleux; Y. Pellegrin; E. Blart, "New photovoltaic devices based on the sensitization of p-type semiconductors: challenges and opportunities." Acc. Chem. Res., 2010, 43, 8, 1063-1071, https://pubs.acs.org/doi/10.1021/ar900275b.
- F. Odobel; Y. Pellegrin; E. A. Gibson; A. Hagfeldt; A. L. Smeigh; L. Hammarström, "Recent advances and future directions to optimize the performances of p-type dye-sensitized solar cells." Coord. Chem. Rev., 2012, 256, 21–22, 2414-2423, http://www.sciencedirect.com/science/article/pii/S0010854512000999?v=s5.
- F. Odobel; Y. Pellegrin, "Recent advances in the sensitization of wide-band-gap nanostructured p-type semiconductors. Photovoltaic and photocatalytic applications." J. Phys. Chem. Lett., 2013, 4, 15, 2551-2564, http://dx.doi.org/10.1039/C1EE01148K.
- E. A. Gibson; A. L. Smeigh; L. L. Pleux; J. Fortage; G. Boschloo; E. Blart; Y. Pellegrin; F. Odobel; A. Hagfeldt; L. Hammarström, "A p-Type NiO-based Dye-Sensitized Solar Cell with a Voc of 0.35 V." Angew. Chem. Int. Ed., 2009, 48, 24, 4402-4405, https://pubs.acs.org/doi/abs/10.1021/jz400861v.
- L. Le Pleux; A. L. Smeigh; E. Gibson; Y. Pellegrin; E. Blart; G. Boschloo; A. Hagfeldt; L. Hammarström; F. Odobel, "Synthesis, photophysical and photovoltaic investigations of acceptor-functionalized perylene monoimide dyes for nickel oxide p-type dye-sensitized solar cells." Energy Environ. Sci., 2011, 4, 6, 2075-2084, https://pubs.rsc.org/en/content/articlelanding/2011/ee/c1ee01148k#!divAbstract.
- A. Maufroy; L. Favereau; F. B. Anne; Y. Pellegrin; E. Blart; M. Hissler; D. Jacquemin; F. Odobel, "Synthesis and properties of push-pull porphyrins as sensitizers for NiO based dye-sensitized solar cells." J. Mater. Chem. A, 2015, 3, 7, 3908-3917, http://dx.doi.org/10.1039/C4TA05974C.
- L. Zhang; L. Favereau; Y. Farre; A. Maufroy; Y. Pellegrin; E. Blart; M. Hissler; D. Jacquemin; F. Odobel; L. Hammarstrom, "Molecular-structure control of electron transfer dynamics of push-pull porphyrins as sensitizers for NiO based dye sensitized solar cells." RSC Adv., 2016, 6, 81, 77184-77194, http://dx.doi.org/10.1039/C6RA15195G.
- L. Favereau; J. Warnan; Y. Pellegrin; E. Blart; M. Boujtita; D. Jacquemin; F. Odobel, "Diketopyrrolopyrrole derivatives for efficient NiO-based dye-sensitized solar cells." Chem. Commun., 2013, 49, 73, 8018-8020, https://pubs.rsc.org/en/content/articlelanding/2013/cc/c3cc44232b#!divAbstract.
- Y. Farré; L. Zhang; Y. Pellegrin; A. Planchat; E. Blart; M. Boujtita; L. Hammarström; D. Jacquemin; F. Odobel, "Second Generation of Diketopyrrolopyrrole Dyes for NiO-Based Dye-Sensitized Solar Cells." J. Phys. Chem. C, 2016, 120, 15, 7923-7940, http://dx.doi.org/10.1021/acs.jpcc.5b12489.
- Y. Farré; M. Raissi; A. Fihey; Y. Pellegrin; E. Blart; D. Jacquemin; F. Odobel, "Synthesis and properties of new benzothiadiazole-based push-pull dyes for p-type dye sensitized solar cells." Dyes Pigments, 2018, 148, Supplement C, 154-166, http://www.sciencedirect.com/science/article/pii/S0143720817314481.
- P. Naik; A. Planchat; Y. Pellegrin; F. Odobel; A. Vasudeva Adhikari, "Exploring the application of new carbazole based dyes as effective p-type photosensitizers in dye-sensitized solar cells." Solar Energy, 2017, 157, 1064-1073, http://www.sciencedirect.com/science/article/pii/S0038092X1730796X.
- M. Raissi; Y. Pellegrin; S. Jobic; M. Boujtita; F. Odobel, "Infra-red photoresponse of mesoscopic NiO-based solar cells sensitized with PbS quantum dot." Sci. Rep., 2016, 6, doi: 10.1038/srep24908, http://dx.doi.org/10.1038/srep24908.
- M. Raissi; M. T. Sajjad; Y. Pellegrin; T. J. Roland; S. Jobic; M. Boujtita; A. Ruseckas; I. D. W. Samuel; F. Odobel, "Size dependence of efficiency of PbS quantum dots in NiO-based dye sensitised solar cells and mechanistic charge transfer investigation." Nanoscale, 2017, 9, 40, 15566-15575, http://dx.doi.org/10.1039/C7NR03698A.
- M. Gennari; F. Légalité; L. Zhang; Y. Pellegrin; E. Blart; J. Fortage; A. M. Brown; A. Deronzier; M.-N. Collomb; M. Boujtita; D. Jacquemin; L. Hammarström; F. Odobel, "Long-Lived Charge Separated State in NiO-Based p-Type Dye-Sensitized Solar Cells with Simple Cyclometalated Iridium Complexes." J. Phys. Chem. Lett., 2014, 5, 13, 2254-2258, http://pubs.acs.org/doi/abs/10.1021/jz5009714.
- F. Légalité; D. Escudero; Y. Pellegrin; E. Blart; D. Jacquemin; O. Fabrice, "“Iridium effect” in cyclometalated iridium complexes for p-type dye sensitized solar cells." Dyes Pigments, 2019, 171, 107693, http://www.sciencedirect.com/science/article/pii/S0143720819301871.
- Y. Pellegrin; L. Le Pleux; E. Blart; A. Renaud; B. Chavillon; N. Szuwarski; M. Boujtita; L. Cario; S. Jobic; D. Jacquemin; F. Odobel, "Ruthenium polypyridine complexes as sensitizers in NiO based p-type dye-sensitized solar cells: Effects of the anchoring groups." J. Photochem. Photobiol., A, 2011, 219, 2-3, 235-242, https://www.sciencedirect.com/science/article/pii/S1010603011000876.
- A. Renaud; B. Chavillon; L. Le Pleux; Y. Pellegrin; E. Blart; M. Boujtita; T. Pauporte; L. Cario; S. Jobic; F. Odobel, "CuGaO2: a promising alternative for NiO in p-type dye solar cells." J. Mater. Chem., 2012, 22, 29, 14353-14356, https://pubs.rsc.org/en/content/articlelanding/2012/JM/c2jm31908j#!divAbstract.
- A. Renaud; L. Cario; P. Deniard; E. Gautron; X. Rocquefelte; Y. Pellegrin; E. Blart; F. Odobel; S. Jobic, "Impact of Mg Doping on Performances of CuGaO2 Based p-Type Dye-Sensitized Solar Cells." J. Phys. Chem. C, 2014, 118, 1, 54-59, http://dx.doi.org/10.1021/jp407233k.
- A. Renaud; L. Cario; Y. Pellegrin; E. Blart; M. Boujtita; F. Odobel; S. Jobic, "The first dye-sensitized solar cell with p-type LaOCuS nanoparticles as a photocathode." RSC Adv., 2015, 5, 74, 60148-60151, http://dx.doi.org/10.1039/C5RA07859H.
- T. Jiang; M. Bujoli-Doeuff; Y. Farre; E. Blart; Y. Pellegrin; E. Gautron; M. Boujtita; L. Cario; F. Odobel; S. Jobic, "Copper borate as a photocathode in p-type dye-sensitized solar cells." RSC Adv., 2016, 6, 2, 1549-1553, http://dx.doi.org/10.1039/C5RA24397A.
- T. Jiang; M. Bujoli-Doeuff; Y. Farre; Y. Pellegrin; E. Gautron; M. Boujtita; L. Cario; S. Jobic; F. Odobel, "CuO nanomaterials for p-type dye-sensitized solar cells." RSC Adv., 2016, 6, 114, 112765-112770, http://dx.doi.org/10.1039/C6RA17879K.
- Y. Farré; M. Raissi; A. Fihey; Y. Pellegrin; E. Blart; D. Jacquemin; F. Odobel, "A Blue Diketopyrrolopyrrole Sensitizer with High Efficiency in Nickel-Oxide-based Dye-Sensitized Solar Cells." ChemSusChem, 2017, 10, 12, 2618-2625, http://dx.doi.org/10.1002/cssc.201700468.
PI: Fabrice ODOBEL
Overview
The possibility to harvest sunlight to activate cheap and stable raw materials such as water, CO2 and N2 to form hydrogen, carbon-based compounds or ammonia and to subsequently use them as fuels or raw commodities for chemical industry is the basement of circular economy. Towards this objective, we aim at designing and synthesizing molecular or hybrid systems made of molecules and semiconductors to mimic the function of the natural photosynthetic apparatus in order to successfully convert sunlight into chemical energy such as hydrogen, carbon monoxide, methanol or ammonia (Figure 1).In collaboration with Stéphane Diring, Metal Organic Frameworks (MOFs) and Covalent Organic Frameworks (COFs) are also developed for solar energy conversion applications (see Photoactive metal-organic framework part).
https://ceisam.univ-nantes.fr/en/imf-team/materials-energy-information-processing/
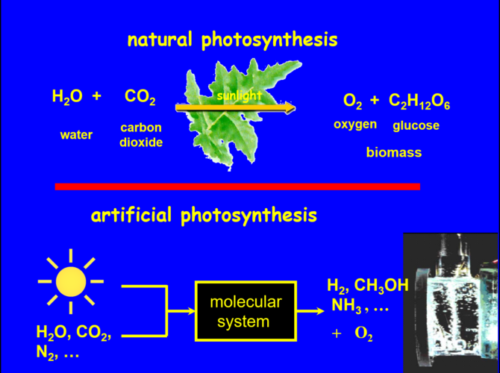
More specifically, our works focus on two main axes: i) the development of molecular systems that reproduce the function of charge photo-accumulation and Z scheme, which represents a rather fundamental approach of artificial photosynthesis and ii) the fabrication and characterization of photocatalytic devices which practically produce solar fuels from water or carbon dioxide. These systems are composed of dye sensitized wide bandgap semi-conductors such as TiO2, NiO to develop dye sensitized photoelectrochemical cells (DSPECs) or dye sensitized photocatalytic systems (DSP). In parallel, we also employ low bandgap semi-conductors such as amorphous silicon and copper indium gallium (di)selenide (abbreviated CIGS) that are coated with molecular catalysts to construct photoelectrochemical cells (PECs) to produce fuels upon sunlight irradiation.
Representative publications of our works
1. Molecular systems for charge photo-accumulation or Z Scheme function
1.1- S. Karlsson; J. Boixel; Y. Pellegrin; E. Blart; H.-C. Becker; F. Odobel; L. Hammarström, "Accumulative Charge Separation Inspired by Photosynthesis." J. Am. Chem. Soc., 2010, 132, 51, 17977-17979, https://pubs.acs.org/doi/10.1021/ja104809x.
In this work, we have designed a hybrid system that couples two consecutive light-induced charge separations, leading to high-yield accumulation of two redox equivalents on single components without sacrificial agents.

1.2- L. Favereau; A. Makhal; Y. Pellegrin; E. Blart; J. Petersson; E. Göransson; L. Hammarström; F. Odobel, "A Molecular Tetrad That Generates a High-Energy Charge-Separated State by Mimicking the Photosynthetic Z-Scheme." J. Am. Chem. Soc., 2016, 138, 11, 3752-3760, http://dx.doi.org/10.1021/jacs.5b12650.
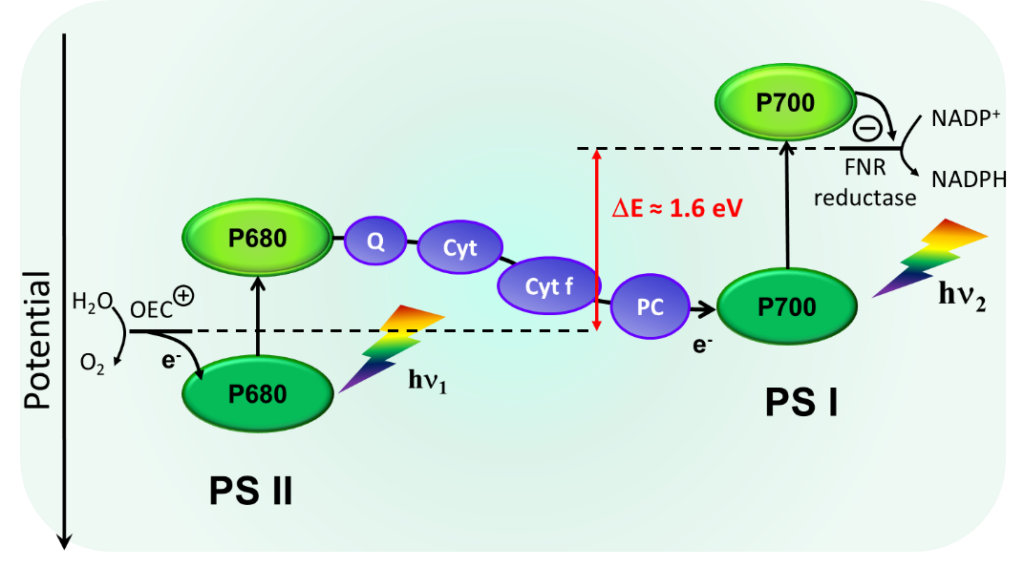
Artificial photosynthesis requires to design a device, which can generate upon light excitation of both a strong oxidant to oxidize water and a strong reductant for proton or carbon dioxide reduction. This is a very difficult endeavor because an excited state generated by a photon from visible light is usually not sufficiently high energy lying to promote both a strongly reducing and strongly oxidizing charge separated state. Accordingly, it is necessary to explore new concepts to solve the aforementioned problem. Our solution is directly inspired from oxygenic photosynthetic system, that is involve two photonic energy inputs via the absorption of two photons by two different photosystems (Figure 3).
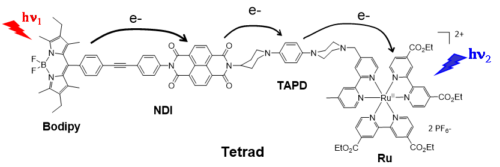
To construct a molecular system mimicking the Z-scheme function driven by two-step photoexcitations, we have designed the Tetrad molecule “Bodipy-NDI-TAPD-Ru” (Figure 4), composed of two different dyes: a Bodipy and a RuII(bipyridine)3, assembled around a naphthalene diimide (NDI) as electron acceptor and a tetraalkylphenyldiamine (TAPD) as electron donor.
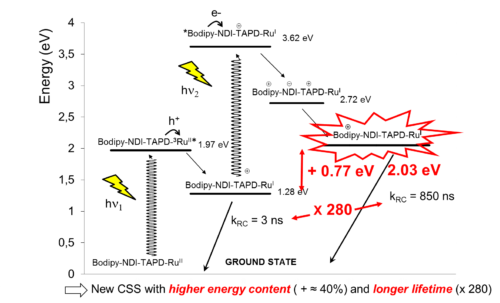
In the above Tetrad, light excitation of Bodipy and Ru complex units ultimately leads to two coupled photoinduced electron transfer events that produces a single charge separated state composed of an oxidized Bodipy+ and a reduced Ru(I) complex (Figure 4). The latter stores a larger energy content than individual photosystem does and provides thus the possibility to generate both a strong oxidant and a strong reductant with two photons of the visible spectrum.
2.Hybrid devices for solar fuels: DSPEC, DSP and PEC
2.1- Dye-sensitized photoelectrochemical cells (DSPECs)
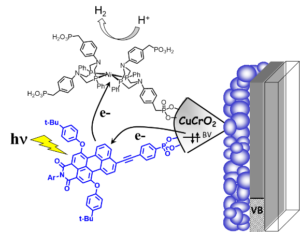
Our long-standing expertise in the field of DSSC (see solar cell projects) enables us to implement the photoelectrodes for DSSCs toward the field of artificial photosynthesis for the development of DSPECs. In particularly, we have searched to exploit the photocathodes to drive the reduction of protons into hydrogen and carbon dioxide to carbon monoxide or the TiO2 based photoanode for alcohol oxidation (Figure 5).
C.E. Creissen; J. Warnan; D. Antón-García; Y. Farré; F. Odobel; E. Reisner, "Inverse Opal CuCrO2 Photocathodes for H2 Production Using Organic Dyes and a Molecular Ni Catalyst." ACS Catal., 2019, 9530-9538, https://doi.org/10.1021/acscatal.9b02984.
E. Nikoloudakis; P. B. Pati; G. Charalambidis; D. S. Budkina; S. Diring; A. Planchat; D. Jacquemin; E. Vauthey; A. G. Coutsolelos; F. Odobel, "Dye-Sensitized Photoelectrosynthesis Cells for Benzyl Alcohol Oxidation Using a Zinc Porphyrin Sensitizer and TEMPO Catalyst." ACS Catal., 2021, 11, 19, 12075-12086, https://doi.org/10.1021/acscatal.1c02609.
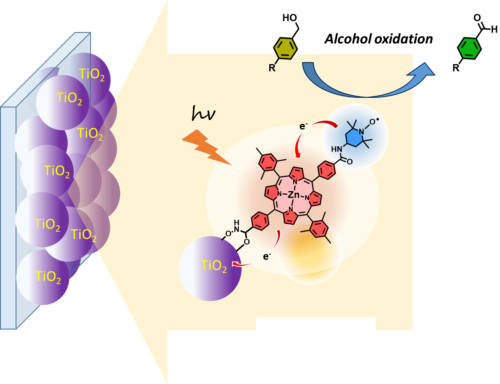
We are very interested to explore other catalytic reactions than water oxidation at the photoanode because water oxidation is a challenging transformation since it involves four oxidizing equivalents. Moreover, it is energetically costing as it requires a potential of 1.23 V vs. NHE at pH = 0 and it is usually catalyzed by expensive noble metal-based catalysts. Finally, water oxidation releases oxygen, which is a product of poor economic value, but they are several attractive alternative reactions that can produce more added values products. In this framework, we have developed the fabrication of a TiO2 based DSPEC using a zinc porphyrin (ZnP) sensitizer and a TEMPO organo-catalyst that quite efficiently catalyzes light driven oxidation of benzyl alcohol derivatives into the corresponding aldehydes (Figure 6).
2.2- Dye-sensitized photocatalysis (DSP) for light-driven hydrogen production and CO2 reduction
Taking inspiration from dye-sensitized solar cells (DSSCs) and our expertise in this area, we embarked in the development of dye-sensitized photocatalysis (DSP) which is composed of a molecular dye and a catalyst co-adsorbed onto a particle of TiO2 whose role is to transport electrons from the dye excited state to the catalyst.
Nikolaou, V.; Charalambidis, G.; Landrou, G.; Nikoloudakis, E.; Planchat, A.; Tsalameni, R.; Junghans, K.; Kahnt, A.; Odobel, F.; Coutsolelos, A. G. Antenna Effect in BODIPY-(Zn)Porphyrin Entities Promotes H2 Evolution in Dye-Sensitized Photocatalytic Systems. ACS Appl. Energy Mater. 2021, 4 (9), 10042–10049. https://doi.org/10.1021/acsaem.1c01975
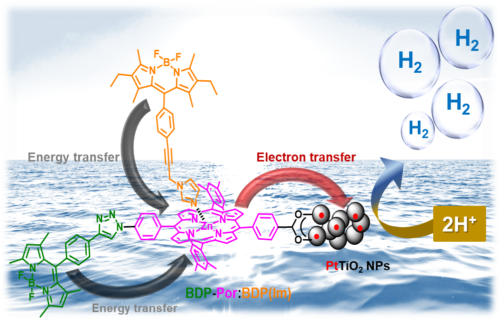
In this work, we have developed DSPs for H2 evolution using BODIPY-(Zn)Porphyrin can amplify light capture of the porphyrin sensitizer by exploiting the antenna effect and thereby increasing the H2 production efficiency (Figure 7).
2.3- Photoelectrochemical cells (PECs)
Inorganic semiconductors and particularly those already used for photovoltaic such as copper chalcogenides are attractive materials to implement into photoelectrochemical cells (PECs) as they can be manufactured at low cost with current industrial processes, they are affordable and very importantly, their conduction band is more negative than the catalytic potential of a wide range of proton and CO2 reduction catalysts.
P. B. Pati; R. Wang; E. Boutin; S. Diring; S. Jobic; N. Barreau; F. Odobel; M. Robert, "Photocathode functionalized with a molecular cobalt catalyst for selective carbon dioxide reduction in water." Nature Commun., 2020, 11, 1, 3499, https://doi.org/10.1038/s41467-020-17125-4.
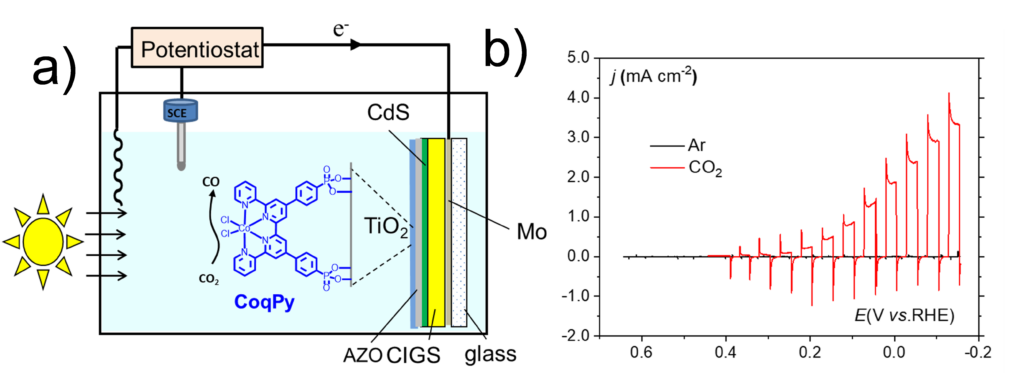
Here, we have developed a photocathode that produces carbon monoxide selectively in aqueous media with high activity, and using earth-abundant molecular catalyst (pH 6.8) is converted to carbon monoxide under visible light illumination with partial current density above 3 mA cm-2 and 97% selectivity, showing good stability over time (Figure 8).
Major collaborations: Prof. Leif Hammarström (Uppsala University, Suède) ; Prof. Eric Vauthey (Université de Genève) ; Prof. Marc Robert (LEM, Université de Paris) ; Prof. Nicolas Barreau (IMN, Université de Nantes) ; Prof. Pere Roca i Cabarrocas (LPICM, Ecole Polytechnique, Saclay) and Prof. Erwin Reisner (Cambridge University).
Financial supports: MolecularZScheme (ANR blanc : 2013-2017); NiOPhotoCat (PSR Région Pays de la Loire: 2012-2015); ClickHybridChem (RFI Lumomat, Région Pays de la Loire: 2017-2020), HybridZScheme (RFI Lumomat, Région Pays de la Loire: 2018-2019) ; PECALO (ANR 2021-2024), ElectroCat (ANR 2022-2026) and Solar-Cat (2022-204, Marie Curie European project).
PI: Stéphane DIRING
Metal–organic frameworks (MOFs), also referred as porous coordination polymers are fascinating highly ordered structures, assembled from metal ions or clusters and organic ligands as building units that can in principle offer such degree of organization. Aside from conventional applications such as gas storage, separation, catalysis, etc., the modularity, nanoscale porosity, enormous chemical variety offered by these hybrid structures also makes them appealing platforms to design and study artificial photosynthetic systems.
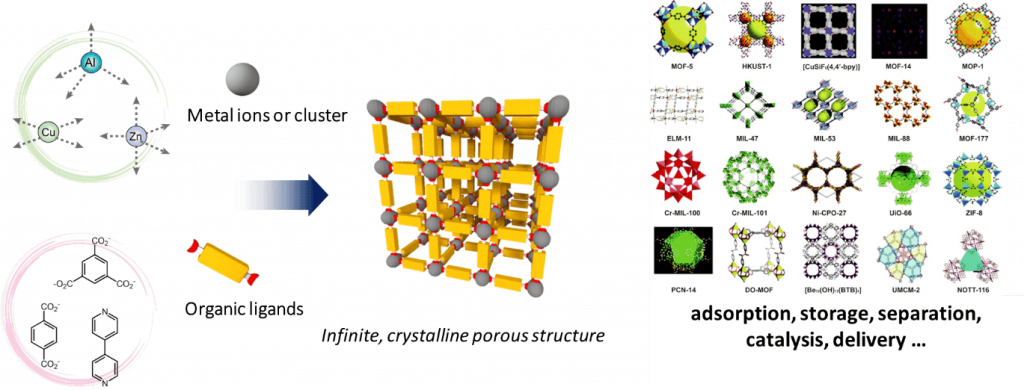
Here, we aim at developing novel photoactive MOF thin films for solar energy conversion. Our approach is based on the controlled and hierarchical integration of multiple photoactive components within the same surface-grown MOF architecture. The main objectives of these new systems are to i) act as panchromatic light-harvesting antennas allowing for directional energy transfer, ii) achieve long-lived charge separated states, and iii) ultimately produce solar fuels, such as hydrogen, or reduce carbon dioxide, by combining these redox potentials with suitable electrocatalysts.
In-depth photophysical investigation will provide a better fundamental understanding of the energy and electron transfer dynamics at play within these hierarchical hybrid materials.
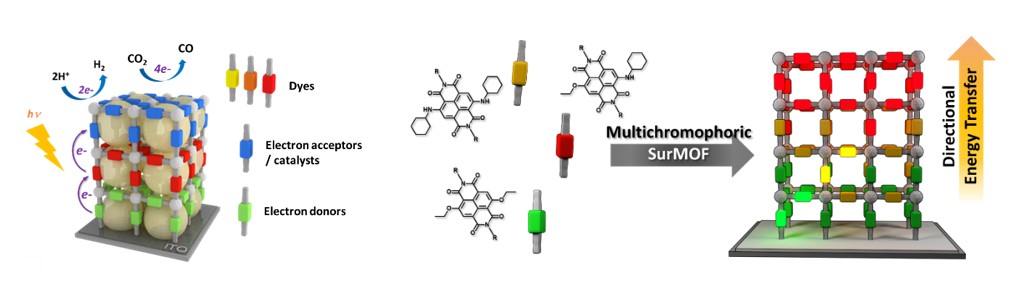
Collaborations
- Christoph Wöll, Ritesh Haladar, Ian Howard. Karlsruhe Institute of Technology (KIT)
- Thoma Devic, Institut des Matériaux Jean Rouxel (IMN, Nantes)
Financements
- LumoMOF (RFI Lumomat, Région Pays de la Loire: 2015-2018)
- PhotoMOF (ANR-JCJC: 2018-2022)
Publications
- A de novo strategy for predictive crystal engineering to tune excitonic coupling; R Haldar, A Mazel, M Krstić, Q Zhang, M Jakoby, IA Howard, BS Richards, N. Jung, D. Jacquemin, S. Diring, W. Wenzel, F. Odobel, C. Wöll ; Nat. Commun. 2019, 10, 2048. (link)
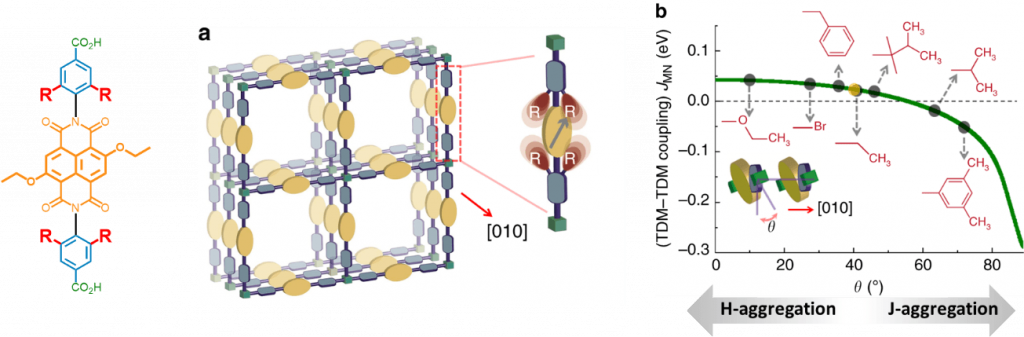
- Crystalline chromophore assemblies render anisotropic energy transfer in metal-organic thin film ; Haldar, M. Jakoby, A. Mazel, Q. Zhang, A. Welle, T. Mohamed, P. Krolla, S. Diring, F. Odobel, B. S Richards, I. A. Howard, C. Wöll :Nat. Commun. 2018, 9, 4332. (link)
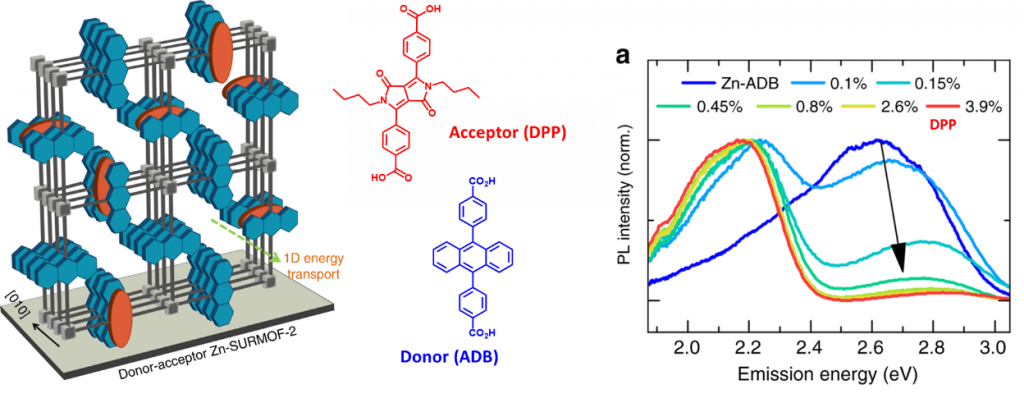
- Enhancing selectivity and kinetics in oxidative photocyclization by supramolecular control ; Haldar, S. Diring, P. K. Samanta, M. Muth, W. Clancy, A. Mazel, S. Schlabach, F. Kirschhöfer, G. Brenner Weiß, S. K. Pati, F. Odobel, C. Wöll ; Angew. Chem. Int. Ed. 2018, 57, 13662 –13665. (link)
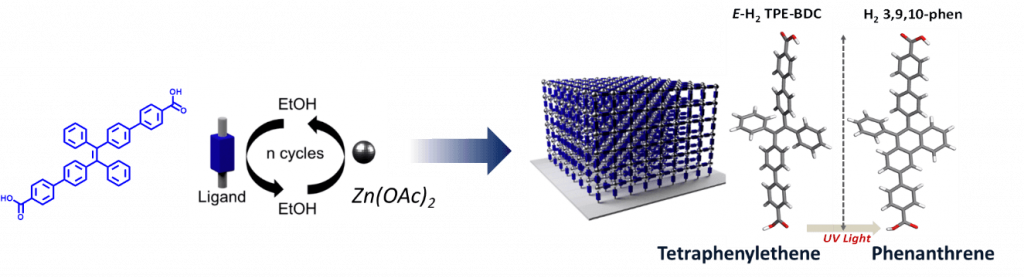
- Excitonically coupled states in crystalline coordination networks ; Haldar, A. Mazel, R. Joseph, M. Adams, I. Howard, B. Richards, M. Tsotsalas, E. Redel, S. Diring, F. Odobel, C. Wöll ; Chem. Eur. J. 2017, 23, 14316. (link)
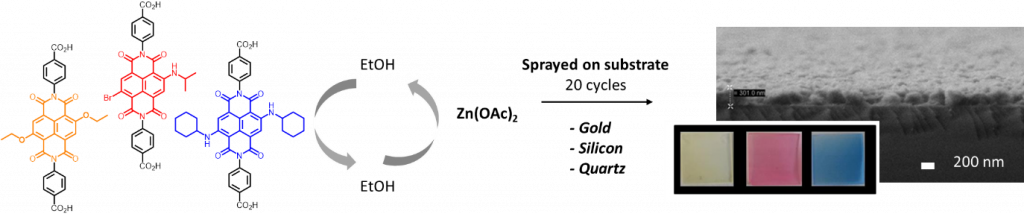
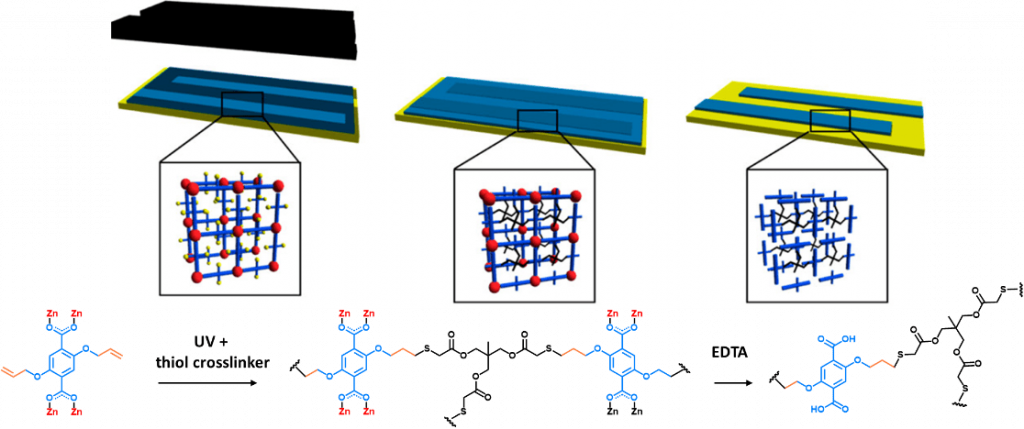
- Localized Conversion of Metal-Organic Frameworks into Polymer Gels via Light-Induced Click Chemistry ; Schmitt, S. Diring, P. G. Weidler, S. Heißler, S. Kitagawa, C. Wöll, S. Furukawa, M. Tsotsalas ; Chem. Mat. 2017, 29, 5982. (link)
PI: Yann PELLEGRIN
Photosensitizers (PS) naturally acquire enhanced reductive and oxidative power upon visible light irradiation: PS* is transiently generated and can either react with an acceptor A (oxidative quenching, OQ) or a donor D (reductive quenching, RQ) generating respectively PS+ and A- or PS- and D+. The corresponding chemical reactions are given below in figure 1:
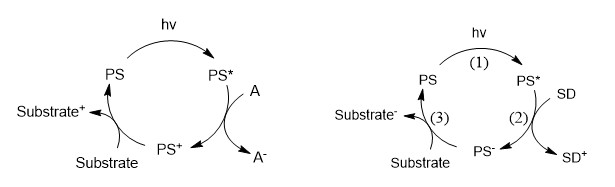
The occurrence of OQ and RQ depends respectively on the photo-reductive and the photo-oxidative powers of PS*. RQ produces PS- which is a more potent reductant than transient PS*. Thus RQ is preferable to reach high reductive power (for activation of reduction catalysts, or simple electron transfer to an acceptor having a deep LUMO. For instance, RQ took place between famous Ru(bpy)32+ and dithio anions1 as D affording Ru(bpy)2(bpy•-)+ featuring a potential of ca. -1.3 V, 500 mV more negative than the excited state oxidation potential of the couple Ru(bpy)33+/Ru(bpy)32+*.
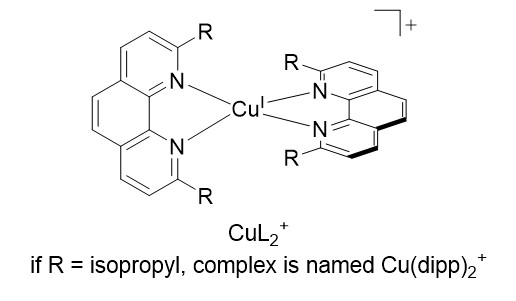
Accordingly, the more cathodic E(PS/PS-), the stronger the reductive power which could be generated with PS* involved in a RQ process. Within this frame, PS chosen as homoleptic Cu(I) complexes CuL2+ (fig 2) (where L is a diimine ligand like 1,10-phenanthroline, bearing bulky substituents R in α of the chelating nitrogens) are very relevant and appealing alternatives to the heavy metal based dyes. Indeed, complexes of the general formula CuL2+ display very similar properties to Ru(bpy)32+ at lower cost and toxicity (MLCT transitions in the visible, the excited state is a charge transfer state, with a decent lifetime).
The relevant photophysical properties of copper(I)-diimine complexes originate in the steric bulk imposed by R (figure 2):2 upon photo-excitation, electron transfer from copper(I) ion to vicinal diimine entails the generation of formally copper(II) species, and therefore a distortion from tetrahedral to square planar geometry which opens several channels leading to a fast deactivation of the excited state.
Most importantly, Cu(I) complexes feature very negative reduction potentials, (e.g. -1.9 V/SCE for Cu(dipp)2+):3 RQ with such complexes, hereafter generically named PSCu, would yield massive reductive power, equal or superior to heavy metal based PS at lower cost. However, RQ with common CuL2+ is a difficult task because they are weak photo-oxidants, namely step 2 in figure 1 is thermodynamically uphill.4
With a view to implementing RQ with common CuL2+ complexes, we focused our attention on the Rehm and Weller equation which states that ΔGRQ (the driving force for RQ) is proportional to -(Ered + E00 – E(SD+/SD) where Ered is E(PSCu/PSCu-), E00 the energy of the excited state and E(SD+/SD) the redox potential associated to the sacrificial donor.
1. Design CuL2+ such that Ered is less cathodic.
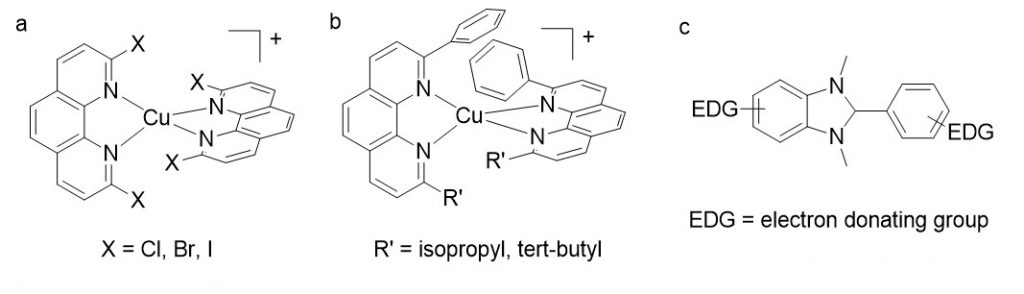
Several complexes have been designed within this frame, by adding electron-withdrawing substituents on the phenanthroline ligand. In particular we prepared a series of complexes where the bulky groups R are plain halogen atoms (figure 3): the idea was to investigate whether halogens were sufficiently encumbering to promote the excited state properties of corresponding copper complexes, and observe the impact on the reduction potential Ered. We showed that the halogen atoms placed in positions 2 and 9 of phenanthroline had a very deep impact on the electronic properties of the resulting copper complexes.5
2. Design CuL2+ such that E00 is increased.
In order to increase E00 without altering the overall redox properties of the phenanthroline ligand, one can simply increase the steric bulk around the copper(I) ion. The stability of the coordination sphere is however at stake (the bulkier the ligands, the more fragile the complexes). In order to get stability and steric bulk, we prepared non symmetrical phenanthroline ligands bearing one ramified alkyl chain in one position, and a phenyl group in the other to implement stabilizing π-stacking interactions. This approach allows to improve by 150 meV the driving force for RQ.
3. Design SD such that E(SD+/SD) is less positive.
In order to qualify as a relevant sacrificial donor in the frame of RQ with copper complexes, the chosen reductant must be irreversibly oxidized at very low potential (ca. 0 V vs. SCE). In order to reach this goal, we focused on the chemical engineering of benzimidazoline derivatives (figure 3c), and modified the molecular skeleton with electron donating groups.
Financials:
- Project PERCO (ANR, 206-2021)
References:
- Cano-Yelo, H.; Deronzier, A. Net photoreduction of tris(2,2'-bipyridine) ruthenium(II) complex in acetonitrile. A photoelectrochemical cell application. Nouveau Journal de Chimie 1983, 7, 147-148.
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and photophysics of coordination compounds: copper. Top. Curr. Chem. 2007, 280, 69-115.
- Garakyaraghi, S.; Crapps, P. D.; McCusker, C. E.; Castellano, F. N. Cuprous Phenanthroline MLCT Chromophore Featuring Synthetically Tailored Photophysics. Inorg. Chem. 2016, 55, 10628-10636.
- Cunningham, K. L.; Hecker, C. R.; McMillin, D. R. Competitive energy-transfer and reductive quenching of the CT excited states of copper(l) phenanthrolines. Inorganica Chimica Acta 1996, 242, 143-147; Cunningham, K. L.; McMillin, D. R. Reductive Quenching of Photoexcited Cu(dipp)2+ and Cu(tptap)2+ by Ferrocenes (dipp = 2,9-Diisopropyl-1,10-phenanthroline and tptap = 2,3,6,7-Tetraphenyl-1,4,5,8-tetraazaphenanthrene). Inorg. Chem. 1998, 37, 4114-4119.
- Brown-Xu, S.; Fumanal, M.; Gourlaouen, C.; Gimeno, L.; Quatela, A.; Thobie-Gautier, C.; Blart, E.; Planchat, A.; Riobé, F.; Monnereau, C.; Chen, L. X.; Daniel, C.; Pellegrin, Y. Intriguing Effects of Halogen Substitution on the Photophysical Properties of 2,9-(Bis)halo-Substituted Phenanthrolinecopper(I) Complexes. Inorg. Chem. 2019, 58, 7730-7745.
PI: Eléna ISHOW
Photochromic organic materials have been known for long for their ability to reversibly photoswitch under light stimulus. Ophthalmic lenses made out of them are the most representative accomplished example, offering unrivalled fast and high-quality eye protection from intense UV sunlight. They have thus stirred ceaseless interest in the field of data storage and optoelectronics to provide high-speed transfer, storage and treatments of optical data, or more recently in organic electronics to photomodulate the performances of the main devices.
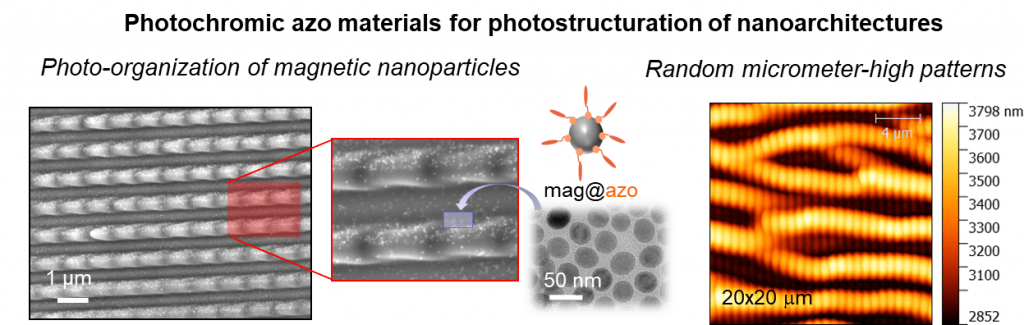
In addition to their photoswitching properties, the class of azo photochromes display remarkable photomechanical properties, yielding fascinating mass transfer and deformation under structured illumination. To exploit such unique properties, we have been developing small molecule-based materials that can originally be processed as nanoparticles or associated to inorganic nanoparticles (gold, magnetic iron oxide) while keeping their photoactivity in the solid state. Our current interests focus on the creation of sub-micrometric patterns with modulated optical or magnetic properties, thereby serving as potential anti-counterfeiting marks.
We are also more recently harnessing such extensive photomechanical properties to elaborate multimodal nanoprobes, stimulating and boosting interests for health applications where the entry of photochromic compounds represents a buoyant field of investigations.
Collaborations :
- Laboratory PPSM, ENS Paris Saclay – GDR CNRS Photo-electrostimulation
- Jean-Luc Duvail et Bernard Humbert (IMN, Nantes) – Raman spectroscopy and magnetism
- Nicolas Delpouve (GPM, Rouen) – Thermal analyses
- Tony Maindron (CEA LETI, Grenoble) - OLED fabrication
- Hiroshi Miyasaka (University of Osaka, Japan) –Femtosecond transient absorption spectroscopy
- Albert M. Brouwer (University of Amsterdam, van’t Hoff Institute, Amsterdam, Netherlands) - Femtosecond transient absorption spectroscopy
- François Lagugné-Labarthet (University of Western Ontario, London, Canada) – Raman and vibrational spectroscopies
- Cleber R. Mendonça (University of São Carlos, São Carlos, Brasil) – Nonlinear optical measurements
References:
- J. Phys. Chem. C 121 (29), 15908–15914, 2017 (link)
Teulle, A. Sancho, E. Ishow, J. Sharma, A. Arbouet, C. Girard, E. Dujardin*
Photochemical imaging of plasmons in multimodal 2D colloidal gold cavities
- ChemPhotoChem 1, 6-11, 2017 (link)
Girard, J. Hémez, V. Silvestre, C. Labrugère, L. Lartigue, J.-L. Duvail, E. Ishow*
Strong color tuning of self-assembled azo-derived phosphonic acids upon hydrogen-bonding.
- Org. Electron. 49, 24-32, 2017 (link)
Olivier, E. Ishow, S. Meunier Della-Gatta, T. Maindron*
Optimization study of inkjet deposition of a hole-transporting small molecule to realize a green top-emitting OLED together with an ald-made thin-film encapsulation.
- ACS Appl. Mater. Interfaces 8 (25), 16207-16217, 2016 (link)
Derue, S. Olivier, D. Tondelier, T. Maindron, B. Geffroy*, E. Ishow*
All-solution-processed organic light-emitting diodes based on photostable photocrosslinkable fluorescent small molecules.
- ACS Appl. Mater. Interfaces, 7 (3), 1932-1942, 2015 (link)
E. Snell, J.-Y. Mevellec, B. Humbert, F. Lagugné-Labarthet, E. Ishow*
Photochromic organic nanoparticles as innovative platforms for plasmonic nanoassemblies.
Funding:
- DGA Tech: Contrat thèse – NAP2 (2018-2022)
PI: Clémence QUEFFÉLEC
Present members: Gennaro PICARDI, François-Xavier LEFÈVRE, Bruno BUJOLI, Clémence QUEFFÉLEC
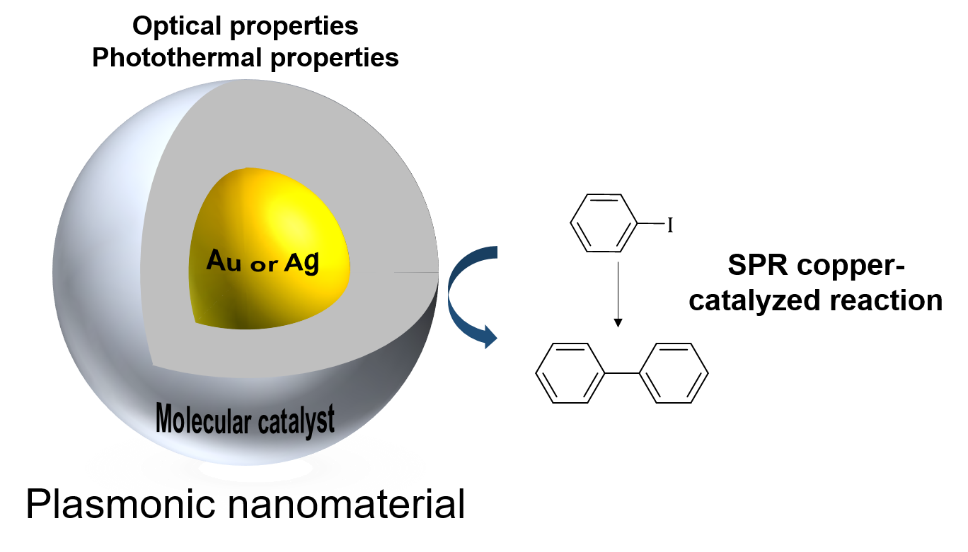
Metallic nanoparticles (NPs) have many useful properties, in particular some NPs have unique optical qualities while interacting with light known as surface plasmon resonance (SPR). Plasmon excitation leads to a range of effects on molecule adsorbed or bound or in proximity to a NP such as thermal effects or charge transfer.
Our main focus is to use the SPR phenomenon for accelerating catalytic reaction rates under laser irradiation using a well-designed molecular catalyst bound to metallic NPs and understand which types of mechanism are occuring.
We have already investigated the Ullmann reaction under laser irradiation. This is a classical example of catalytic carbon-carbon bond forming process, based on the coupling of aryl halides mediated by copper. The hybrid nanocomposites we synthesized were proven to be efficient in accelerating the rate of the latter reaction under light irradiation.
We focused our investigation as well on catalytic hydrolysis of methyl parathion (MeP). MeP is a compound used in large-scale as pesticide and insecticide, but is a nerve agent obviously dangerous to the health.
We are currently investigating other copper-catalyzed challenging reactions with different types of molecular catalysts bound to different type of metallic NPs.
Collaborations :
- B. Humbert and JY Mevellec (IMN, Nantes University, France),
- M. Lamy de la Chapelle (IMMM, Le Mans University, France),
- Nordin Felidj (Itodys, Paris University, France),
- Claire Mangeney (LCBPT, Paris University, France),
- D.A. Knight (Florida Institute of Technology, Florida, USA).
Financements :
- Région Pays de la Loire (Pari Scientifique X-TREM),
- CNRS (Projet PICS COSMOCAT).
References:
- Forato F.; Talebzadeh, S.; Bujoli, B.; Queffélec, C.; Trammell, S.A.; Knight, D.A. “Core-shell Ag@TiO2 nanocomposites for low-power blue laser enhanced copper(I) catalyzed Ullmann coupling” ChemistrySelect 2017, 2, 1-6.
- Talebzadeh, S.; Forato F.; Bujoli, B.; Trammell, S.A.; Grolleau, S.; Pal, H.; Queffélec, C.; Knight, D.A “Non-photochemical catalytic hydrolysis of methyl parathion using core-shell Ag@TiO2 nanoparticles” RSC Adv. 2018, 8, 42346-42352.
- Forato F.; Talebzadeh S.; Rousseau N.; Mevellec J.Y.; Bujoli B.; Knight D.A.; Queffélec C.; Humbert B. “Functionalized core–shell Ag@TiO2 nanoparticles for enhanced Raman spectroscopy : a sensitive detection method for Cu(II) ions” Phys. Chem. Chem. Phys. 2019, 21, 3066-3072.
- Talebzadeh S.; Queffélec C.; Knight D.A. “Surface Modification of Plasmonic Noble Metal – Metal Oxide Core-Shell Nanoparticles” Nanoscale adv. 2019, 1, 4578-4591
- Queffélec, C.; Forato F.; Bujoli, B.; Knight, D.A.; Fonda, E.; Humbert, B. “Investigation of copper oxidation states in plasmonic nanomaterials by XAS and Raman spectroscopy” Phys. Chem. Chem. Phys. 2020, 22, 2193-2199.
- Gennaro P., Humbert B., Lamy de la Chapelle M., Queffelec C. “Surface Modification of Au Nanoparticles with Heteroleptic Cu(I) Diimine Complexes” J. Phys. Chem. C 2020.
