Prof. James Mayer is a specialist of electrocatalysis, photoinduced electron transfer, proton coupled electron transfer; see his WEB page: https://mayerlab.chem.yale.edu
At this occasion, he will be hosted by us and he will give a talk on Monday April 29th at 14h30 in Marie Curie room on the topic” Proton-coupled electron transfer at interfaces: metals, metal oxides, and silicon”.
University of Nantes
29 April, 2024
James Mayer
Yale University, james.mayer@yale.edu
Proton-coupled electron transfer at interfaces:
metals, metal oxides, and silicon
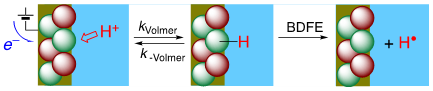
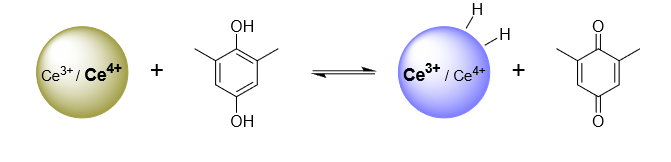
Hydrogen is a ubiquitous component of our environment, of most interfaces, and of many catalytic processes. A hydrogen atom is a proton and an electron, so the thermochemistry of H transfer and coupled 1H+/1e– transfer (PCET) are the same (± a constant). The electrochemical potential of the Volmer reaction (eEVolmer) is thus equivalent to the surface–H bond dissociation free energy (BDFE; top image below). Using both electrochemical and equilibration methods, our laboratory has extended these long-known principles to NiO electrodes, colloidal TiO2, IrO2, and CeO2-x and Au nanoparticles (schematic below), and silicon surfaces. These studies show that PCET is the dominant redox reactivity at all of these solid/liquid interfaces, with buffered aqueous and non-aqueous solutions. Some common themes are emerging for these quite disparate materials, with widely varying electronic structures. The commonalities start from the conclusion that the surface–H BDFEs are the primary determinant of surface reaction thermodynamics and kinetics (versus, for instance, the Fermi level of the electrons in the material). The BDFEs and reduction potentials vs. RHE seem to be independent of solvent and buffer, and they often vary substantially with surface coverage (non-ideal behavior, not following a Langmuir isomer). These observations have important implications for the redox reactivity of interfaces.